CHEMICAL COMPOSITION OF BODY
CHEMICAL COMPOSITION OF BODY – The human body is made up of many types of chemical substances made up of about 24 elements. About 98% of the mass of the human body is made up of these 6 elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. And at approx. 0.85% is composed of the other five elements: potassium, sulphur, sodium, chlorine, and magnesium. All 11 are necessary for life. These chemical substances are mainly of two types – INORGANIC AND ORGANIC SUBSTANCE.
INORGANIC SUBSTANCES
THESE are those substances in which carbon element is not found. The molecules of these substances are relatively small molecules of organic matter in which the atoms are mostly connected by electrically bonded bodies. The body’s water, acids, and salts are inorganic substances.
There are many more life-useful substances in the body that fall under the category of inorganic substances.
Water: –
Chemical condensation of water is h2o. It is formed by the association of hydrogen and oxygen elements, which is 70% of the total body weight. Life is not possible without it. Hence, it is a life-long inorganic substance. It has the following characteristics: – Water: – is a neutral fluid at physical temperature.
- It is clean, colourless, and odourless.
- The pH value of pure water is 7.
- The boiling point of water is 100 ° C.
- The freezing point of water is 0 degree C.
Chemical composition of the body– Water is found in all three forms of matter: solid, liquid, and gas. Water in solid form is called ice. Water turns into ice at zero degrees heat. Water in gas form is called water vapour. On heating, the water starts turning into vapour and at 100 degrees Celsius, it starts boiling. Water remains in a liquid state between freezing and boiling temperatures. It is a natural and highly available universal solvent
FUNCTIONS:– Water acts as a solvent for life-useful substances in the body. It is also called the universal solvent because most of the matter dissolves in it and is found in all places.
- All biochemical reactions useful in life are carried out only through water.
- When water evaporates from the skin, it reduces body temperature, that is, it cools the body. This helps keep the body’s normal temperature constant at 98.6 degrees Fahrenheit in a cool hot environment.
- Water in body tissues and cavities reduces the risk of trauma.
- Water carries gases and waste materials from one place to another place in the body.
- Most of the body’s acids, salts, salts, etc. dissolve easily.
- Water helps in digestion of food by hydrolysis
ACID:
Acids are chemical inorganic substances that dissolve in water and release hydrogen particles, which can be measured by the instrument; they are also called proton donors. Other characteristics of acids are: –
It is sour.
Chemicals and salts are made by chemically reacting with alkalis.
The pH values of acids range from 0 to 6.99.
It digests and sterilizes food.
SULFUR ACID: –It is also a strong acid that is not found in the body, its chemical formula is to h2so4
NITRIC ACID: –Nitric acid is also a strong acid but it is not found in the body, its chemical formula is HNO3
CARBONIC ACID: –IT is a weak acid that does not break down into fully charged particles. It is formed by the combination of carbon dioxide and water in the cells and fluid of various organs. Its chemical formula is H2CO3. It helps in stabilizing the acidity and alkalinity of the blood. This acid is synthesized and dissolved in the removal of carbon dioxide.
ALKALIS are chemical substances that, when dissolved in water, give hydroxyl ions, which can be detected by any device or device. They are also called proton receptors. Among its other features are: –
It is astringent and smooth in taste.
Their pH values range from 7 to more than 14.
By making a chemical reaction with alkali acids, salts, and water are made.
SALTS: –Salts are chemical inorganic substances produced by the chemical reaction of acids and bases. They break down easily in water and give cation and anion.
Perform biological functions in the human body. Their other features are: –
1.They is pungent in taste.
2. These salts are also called electrolytes.
Chemical composition of the body
ORGANIC SUBSTANCE: –
The substances in which the carbon element is found are called organic SUBSTANCE. Along with carbon, hydrogen element is also present in them. The molecules of these substances are very large.
The important organic substances of the human body are fat, carbohydrates, proteins, nucleic acids, and ATP.
PROTEIN:–
Protein is the most essential and primary organic substance of the body. It is made up of 20 amino acids. 20 types of amino acids are needed for the human body to form proteins. All amino acids are supported by an amine group and an acid group. These amino acids join together to form a long chain. One type of protein is a long chain of amino acids ranging from 50 to thousands.
Proteins always consist of elements of carbon, hydrogen, oxygen, and nitrogen. Some proteins are also made up of elements like sulphur, phosphorus, and iron. This protein is found inside the body in the form of enzymes, hormones, antibodies, haemoglobin, etc. Nuclear proteins are found in the centre of cells in the form of DNA and RNA.
A polypeptide is a low-molecule substance whose water decomposition produces two or more amino acids. Dipeptides make 2 molecules of amino acids.
[full form of ATP is Adenosine triphosphate]
Adenosine triphosphate ATP: –
It is a small organic material containing three phosphate molecules, which stores and releases energy. Its chemical structure is made up of adenine and ribose sugars. Huge reserves of energy are hidden in dams made with first, second, and third phosphate molecules.
Carbohydrates: –
The substances made from glucose and glucose series are called carbohydrates. This body is a very useful organic material, which contains carbon, hydrogen, and oxygen elements. Carbohydrates are energy-producing foods in the body. Carbohydrates are of the following types: –
Monosaccharides: –
Those sugars which contain one molecule of glucose or fructose or galactose in one molecule are called monosaccharides. Or we can say two monosaccharides joined covalently like if we take sucrose it is (glucose + fructose). These simple sugars in the body are glucose, fructose, and galactose types.
Disaccharide: –
If one molecule of glucose is made up of two molecules of glucose, then this type of sugar is called a disaccharide. Sucrose, maltose, lactose, etc. are useful body sugars.
One molecule of sucrose contains one glucose molecule and the other fructose molecule. Similarly, lactose sugars contain one molecule of glucose and galactose. One molecule of maltose sugars is made up of two glucose molecules.
Nucleic acids: –
Those proteins which are found in the centre of the cell are called nuclear acids. There are two types of DNA and RNA.
Nucleic acids are the largest organic protein molecules in the body whose chemical composition includes nitrogen-based alkali, sugar, and phosphate groups.
These organic substances of the body are high sources of energy, they are also called lipids.
The chemical organization of lipids is made up of elements of carbon, hydrogen, and oxygen, but elements such as phosphorus and nitrogen are also found in them.
The major lipids of the body are triglycerides, phospholipids, steroids, lipoproteins.
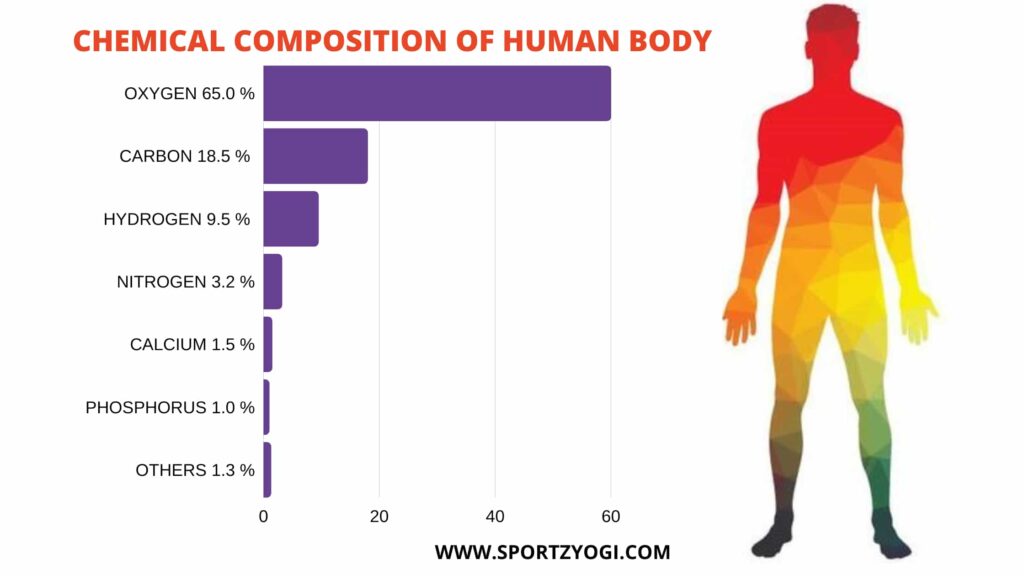
Chemical composition of the human body are:-
Oxygen-65% Sulfur-0.3
Carbon-18.5 Sodium-0.2
Hydrogen-9.5 Chlorine-0.2
Nitrogen-3.2 Magnesium-0.1
Calcium-1.5 Iodine-0.1
Phosphorous- 1.0 Iron- 0.1
Potassium -0.4 Everything else-0.1
READ MORE ABOUT -: CLASSIFICATION OF THE BONES IN THE BODY
https://www.sportzyogi.com/classification-of-bones-in-the-human-body/